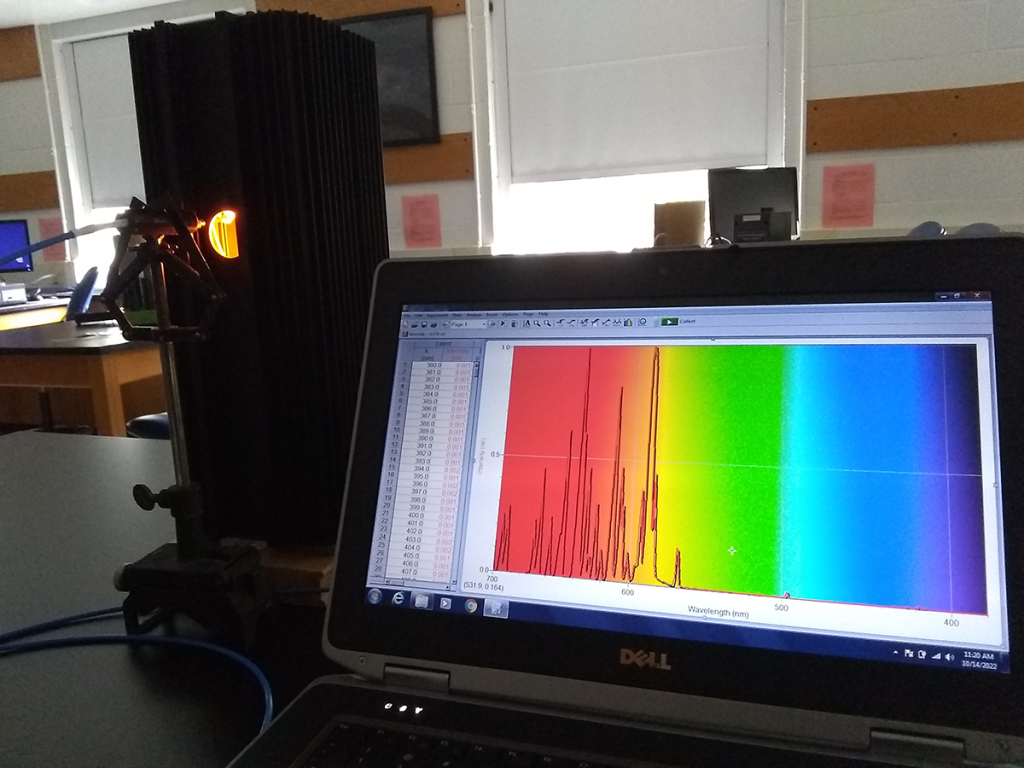
Astronomy is roughly 98% figuring out how to look at stuff better than our eyes can do it.
Gathering more light with large-aperture lenses and reflectors. Gathering more light with long camera exposures. Using detectors for light outside the visible spectrum, from radio waves to gamma rays. Launching telescopes a million miles into space to get away from our pesky atmosphere. Splitting the broadly blended colors we perceive into their component wavelengths.
That last one’s the easiest to accomplish in a student lab setting, and it’s a broadly useful scientific tool across many disciplines. Turns out that certain particular constraints caused by quantum physics make all sorts of other observations possible. Who knew?
Pictured above is a low-pressure sodium lamp, just like the ones that once illuminated nighttime streets around the world with their flattening orange glow. Looks orange to our eyes, but it’s primarily a mix of red, orange, and yellow wavelengths. If you measure those carefully enough, you can discern a certain “fingerprint” on a spectrum of light that would tell you if sodium is or isn’t present in what you’re observing.
Same applies to hydrogen and helium. Nitrogen and oxygen. Argon and neon. Carbon dioxide. Water. Every atom and molecule – including different ionized states, which is a particularly useful bit of information for astronomers – has its own unique spectrum of light it emits. You just need to look at it in the right way.
Even if it’s millions of light-years distant.