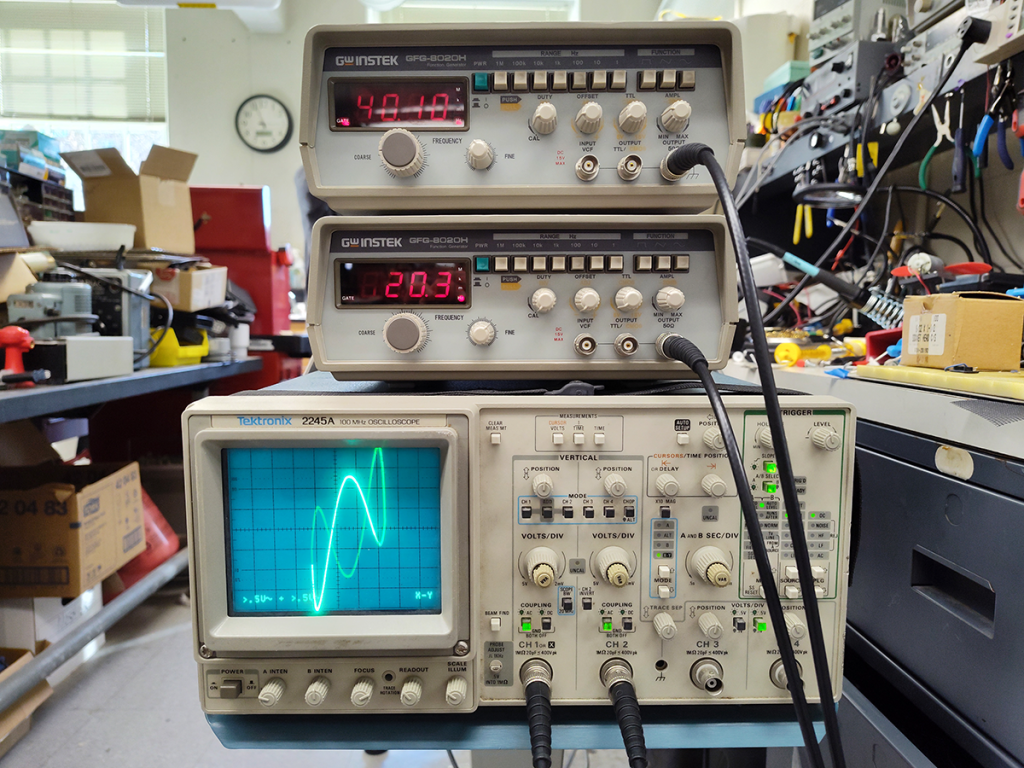
Take two function generators, an old CRT oscilloscope, a couple of power and BNC cables, and look! Whirling, dancing lissajous figures!
Chunky knobs! Clicky buttons! Drifty outputs! Squiggly curves!
Discoveries in the Physics & Astronomy shop | Science, curiosities, and surprises

Take two function generators, an old CRT oscilloscope, a couple of power and BNC cables, and look! Whirling, dancing lissajous figures!
Chunky knobs! Clicky buttons! Drifty outputs! Squiggly curves!

Need a thing, but can’t get it in the right size, right shape, right odd set of dimensions? That’s one reason to keep a workshop in the basement. If we can possibly make it, we’ll certainly try.
Pictured: a custom optics breadboard, for a very specific apparatus, with many, many drilled, tapped, and cleaned 1/4″-20 mounting holes. It’s big, and shiny, and has a bright future ahead!
Probably with lasers or something. Lots of lasers around here.

The color for next semester’s toy kits: violet.

Look, sometimes the very specific version you want only exists in a format that’s hard to make use of, like a VHS cassette. That’s why we have helpful, talented people with specific sorts of tech savvy to help us out with bringing oldies but goodies into a more modern format that’s still destined to veer into obsolescence after a while. Thanks, Wes!
For those wondering, AAPT stands for the American Association of Physics Teachers.
In this case, anyway. But for a thought experiment, imagine the collapse of Galloping Gertie as narrated by these other AAPT organizations and/or acronym users:
Some of those could be really entertaining. Just don’t mention poor Tubby the cocker spaniel to the animal assisted play folks.

In our Physics & Astronomy labs, we use metersticks with great frequency. Often for measurements, sometimes to approximate distances that make the arithmetic easier, and occasionally as a handy tool for pointing to the projector screen.
They aren’t super-high precision any more than the rulers you remember from elementary school, and for that we have other tools. Sometimes, as you can see above, the years have warped and twisted things a bit. We adjust.
As you might expect, they offer metric distances on one side, inches and feet on the other. The best ones – the oldest set – were long ago painted black to conceal those SAE units. Clearly, someone grew weary of students measuring everything in inches and then complaining that the math wasn’t working out right.

Physics doesn’t rely on field work as much as some other disciplines – biology, geology – but sometimes it’s necessary and the fresh air does us good. So do the occasional wild treats found along the trail.
Just be sure to share them with the local bears and birds!


In great big boxes full of boxes, the toys begin to arrive. We stash them in corners, in front of other shelves, any place mostly out of the way before separating, sorting, packing, and distributing.
Three hundred yo-yos, Imperials and Butterflies, in an assortment of colors. Every box is full of surprises!

It’s July, and that means it’s the time of year for restocking on toys! Bouncy balls, suction cup blowdart guns, silicone poppers, the works.
This is all for advanced scientific education, mind you. Important stuff, building a better tomorrow, etc.

Remember slide film? Carousels and projectors and hauling out the big screen to see those vacation photos? Are you old enough to remember high school and/or college lectures on slides? The shop techs remember.
Nowadays everyone’s much more likely to use Slides than slides, of course. More portable, for the most part. Easier to edit, up until the last moment. Overall, a lot of advantages. But the old-school ones were pretty cool, too.
One can only hope that back in the days of the Audio-Visual Aids Department (we’re assuming they’ve been subsumed into L&IT, but not ruling out the possibility of a now-defunct academic department), they wheeled these – and film projectors, and VCRs, and hopefully LaserDiscs, too – into your classroom space on the classic steel cart. Embedded YouTube clips just aren’t the same.

Much of our day revolves around the Physics and Astronomy labs in Olin Science Building, which was the finest construction the budget could shoulder in 1954.
Which, after 7 decades, some renovations, and who knows what else, means there are a few minor holes here and there.
Holes which act like a pinhole camera in an otherwise dark lab, casting images of the sun and tree branches on the floor.