
Science: if we can learn something from sending a 1,200 lb. payload over Saskatchewan via (a very large) balloon, by golly we’re going to try.
Discoveries in the Physics & Astronomy shop | Science, curiosities, and surprises

Science: if we can learn something from sending a 1,200 lb. payload over Saskatchewan via (a very large) balloon, by golly we’re going to try.

An electronic buzzer buried inside a foam ball, on a long cable with a switch and handle at the end. Flick the switch, and a piercing 2,500 Hz signal begins. Whirled in a big circle around your own head, the tone persists. For everyone else in the room, it creates a cyclic Doppler shift, a repeating weeee-oooo, weeee-oooo that sticks in the brain even after it’s done, like when you see phosphene images behind your eyelids after catching a glimpse of something way too bright.
We can’t overstate the wonderful modifications to the original object, back in 2010, which added the cable and switch. Your bog-standard Doppler ball – available from several scientific apparatus purveyors – requires one to open up the ball, turn on the buzzer, then close it back up. Tossing it back and forth between students illustrates the concept.
Then, when you’ve had quite enough and just want it to stop, you have to pry it open and shut the whole thing off. (This vintage version, pre-mod, required full-on battery removal.) The Doppler ball: it teaches us all kinds of new lessons!

Camping. For science!
The enormous beige box is a “Basecamp Kitchen Kit,” in case you’re wondering. It’s half as heavy as it looks.

Summer is progressing quickly, and it won’t be long before it’s toy kit time once more, including this multicolored assortment of silicone poppers! Available in different colors and sizes, over time you learn which ones pop the best.
Marbled performs better than solid colors.
Pink is often the best. A good one can nearly slap the ceiling from bench height.
No certainty as to why. But they sure are fun!

When you need to keep things cold, you have options, depending on your temperature needs and what’s available. Carnot cycle refrigeration is handy, effective, and reversible when you need to supply heat (think a heat pump or household refrigerator). You can use the thermoelectric effect via a Peltier device, in which electric current through dissimilar materials causes heat energy to flow in one direction. Or you can just huck a bunch of cold stuff at your target and wait for thermal equilibrium.
Cool running water is remarkably effective in this case. Even colder: ice, though a slurry of ice and water is often faster because of the increased convection and heat transfer through the cold liquid. Sometimes that sizable dollop of energy required for phase transition is really handy! Salt/ice/water slurry gets you colder still, and it’s a great way to make ice cream in the backyard.
Colder still: dry ice, or solid carbon dioxide. (We’re now at the point where you really don’t want this stuff to get on your bare skin.) It has some limited cooling potential, as it sublimates directly to gas at atmospheric pressure, and so good contact and heat transfer can be slow. You can get it to liquid form – the correct cold temperature range and high pressure – which is how we make dry ice when we need it. If you need a suitable liquid for extra-cold chilling, you’re probably looking at liquid nitrogen.
At -77°C, it’s very cold. Your chilling rate becomes limited by the Leidenfrost effect – that thing where a layer of insulating gas forms between the hot and cold materials, thermally and spatially separating them. Same thing happens when you dip a wet finger into a vat of molten lead, or the slippery skitter of a water droplet across the surface of griddle heated just right for pancakes. But, still, cold. Very cold. We use it as a backup for a freezer that’s supposed to hang out at -80°C as long as the power’s on. The stuff inside can handle slightly “warmer” temperatures for the time it takes to repair a power outage.
If you need even colder? Really dedicated folks dial it up to 11 and use liquid helium. That’s a whopping -269°C, which sounds intense. Or, if you prefer, about 4 K. It gets used here at the University, just not in Physics. (Astronomers might study it, but at the safe distances used for telescopic observation.)
All of these extra-cold normally-gases need special handling and care, not only for the temperature concerns, but also for what happens when they warm up and expand and displace all of the breathable air around. (Bad.) We all have some sense of the too-much-carbon-dioxide/monoxide symptoms – sluggishness, blue lips, etc. – but those of us not trained as fighter pilots aren’t as readily aware of the distinct symptoms of too much nitrogen, not enough oxygen. If you are one of the lucky few, you get to undergo normobaric hypoxia training, which can lead to roughly 18 seconds earlier awareness of hypoxia, which sounds like an awful lot in a plane that can travel a third of kilometer in that period at Mach 1.
As for the the dangerous effects of breathing a sudden roomful of now-gaseous helium? We assume your final words are hilariously high-pitched.

Behold: a box which counts! That’s it, for the most part. It counts pulses of positive voltage. Very quickly, and you can set some thresholds to tell it to count certain values but not others.
It also gates over an interval you set, so you can tell how many pulses it receives over, say, one second. It counts, displays the total, then counts again. Displays the new number.
We use these for our wave/particle duality lab experiment, which relies on counting individual photons. Yes, those. The teeny, massless quantum packets of energy, the messenger particles of electromagnetism. Light. It acts in non-intuitive ways, and the students who think “that’s amazing and I want more!” sometimes become Physics majors.
Part of using this box – just one aspect – is helping convince those students that only one photon at a time can be reaching the photomultiplier tube sensor. At the speed they move, a mind-boggling number of photons can zip through that meter-long box without bunching up. c in air isn’t all that far from c in a vacuum, so if your one-second counts aren’t remotely near 299,792,458 (adjusted for PMT sensitivity and other losses), you know some of those photons are pretty lonesome. Sometimes you need a little math to make sense of things you can’t directly sense.

One other fun aspect is a little switch hidden on the back: cricket. It’s the volume switch, letting the box emit a little beep for every pulse it counts.
If you’re counting pulses from a radioactive source, which arrive randomly, it can be informative to hear these irregular signals, gated and grouped into numbers which show a decaying curve.
If you’re counting 100,000 photons every second, in a room of other lab benches also counting thousands of photons? Less informative, more irritating.

Sometimes, we have old equipment which is rarely, if ever used. Case in point: the mid-20th-century spectroscopes which have been supplanted by digital spectrometers. They’re both effective tools for examining a spectrum of light, one by eye and the other fed by a USB cable. Using a diffraction grating, they split light into its constituent spectrum – its rainbow, more or less – and can identify the presence of individual wavelengths. Not something our eyes can do, as they blend everything together, though that’s very helpful in most situations, such as reading this on your screen.
Summing bands of reddish, greenish, and bluish into a broad rainbow of colors is one neat-o trick.
With a diffraction grating, reflection grating, or prism, you can refract light out along a range of angles which correspond to its constituent wavelengths. Put a sensor at a known angle – your eye or a semiconductor exhibiting the photoelectric effect – and you know the wavelength if you sense a photon. It’s a simple piece of information which can be used to unlock a staggering amount of interesting, related information about what you’re observing.

You can also use a diffraction grating to get a quick sense of the entire visible spectrum of a source by holding it off to the side. Remember: the angle of the light’s path change as it refracts, so you’re trying to angle it back to your eye. Hydrogen has a distinctly pinkish-purplish hue when excited at high voltage, and you can see the dominant red and blue lines in its spectrum. With just that one electron to absorb energy and emit photons, the spectrum can only be so complicated.

That’s in contrast to helium, with its two electrons. The spectrum doesn’t look white, per se, but is much more filled out than hydrogen. Look at those spectral lines, and there are so many more! They’re distinct, measurable, and provide a “fingerprint” that can be immensely useful for scientific study. Or for just looking cool.

‘Tis that most joyous of days in the beginning of the semester: physics toy kit day! A bag full of odds and ends, perfect for playing, experimenting, and providing tactile bits to use when working through physics problems. Batteries, compasses, various wires, polarizing filters, nails, magnets, and balloons. Always balloons.
Each kit contains two small neodymium magnets, because magnets are amazing. First, you’re bound to stick them together, then spin one around and feel them repel. Surprisingly strong such wee little cylinders. Then check what they stick to around you: whether or not they feel attracted to stainless steel is always intriguing. (The answer is: depends on the type of steel and how it was formed.) Stick them together across a string and let it hang: you’ve built a compass!
Pay attention to the time and location of the sun – or Polaris if you’re pulling an all-nighter – and you can tell which pole of your magnets is which. Maybe it’ll come in handy?
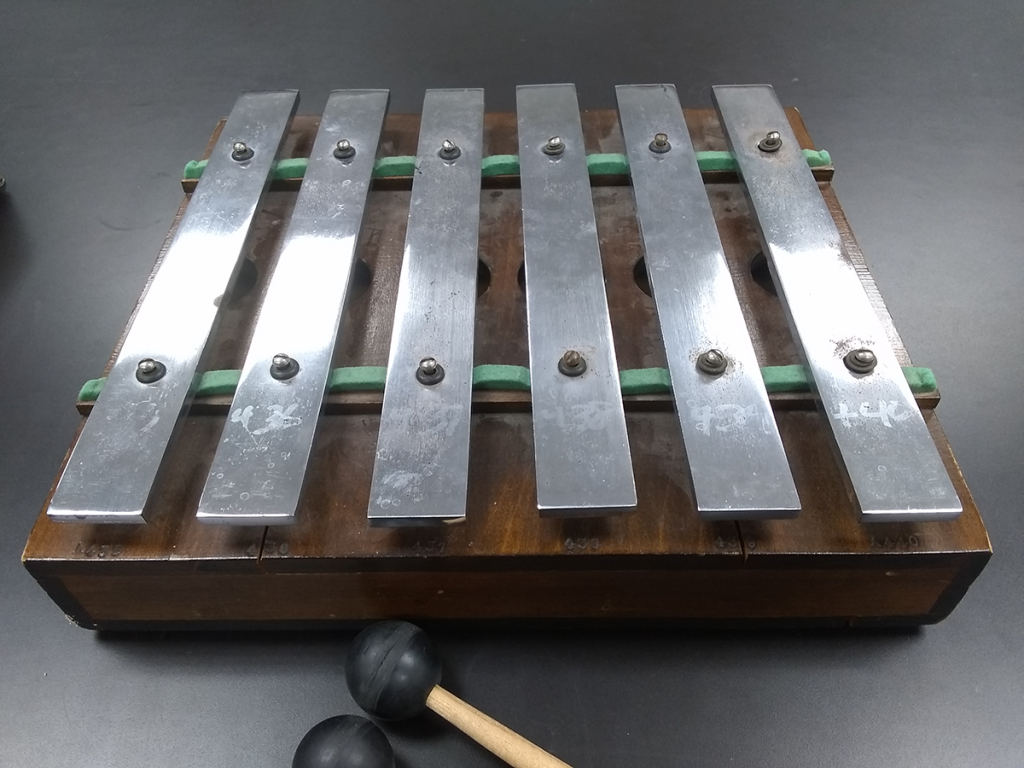
In acoustics, there’s a phenomenon known as beats, which is when two similar tones generate an interference pattern that sounds like a pulsing beat. It happens with waves of all kinds, waves being moving energy and all that, but sometimes it’s easier to really get the sensation when you hear it. Graphing out the sinusoids and showing the constructive and destructive interference helps explain it. Hearing that wubwubwubwubwub cements it.
We have what looks, at first glance, like a glockenspiel, with its metal bars all in a row. Tap one with the mallet, and it sounds almost the same as the one next to it. Almost. Tap two at once, and you get the beats.
At one end, it’s 440 Hz. Then 439, 438, 437, 436, and 435 Hz. Not only can you hear the beats, but you can very clearly hear the change in beat frequency as you combine tones in different combinations. It’s very cool.
Also quite unnerving after a while. woobwoobwoobwoobwoob
Physicists don’t particularly like bare concrete block walls any more than the rest of us. Lasers: available in a rainbow of eye-searing colors!
(Always practice safety when using lasers.)