
“Connerctor!”
Discoveries in the Physics & Astronomy shop | Science, curiosities, and surprises

“Connerctor!”

In great big boxes full of boxes, the toys begin to arrive. We stash them in corners, in front of other shelves, any place mostly out of the way before separating, sorting, packing, and distributing.
Three hundred yo-yos, Imperials and Butterflies, in an assortment of colors. Every box is full of surprises!

‘Tis the season for PHYS 211 toy kits!
A bag full of goodies for each and every student studying classical and modern physics this upcoming semester. Yo-yo, fidget spinner, bouncy balls (large and small), rubber ball on string, silicone fun poppers (large and small), metal coil spring (not a Slinky, but really it’s a Slinky), and a pair of balloons. Drinking birds and blowguns (not pictured) to be distributed later in the semester.
For those wondering: the big bouncy ball is way better than the little one. Same goes for the fun poppers. The little ones hop a bit, while the big ones bounce all over the shop. You know, for science.
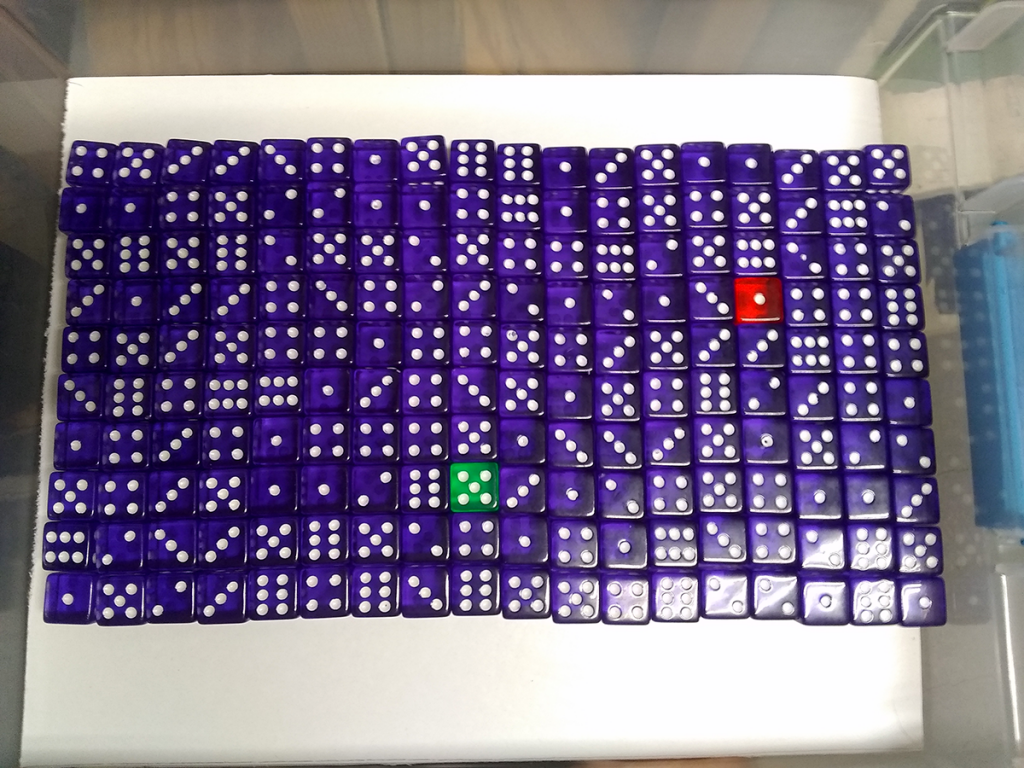
Dice! Bins of colorful dice, each with 178 of one bold color, plus two going their own way. Each bin arrayed in a 10 x 18 or 12 x 15 grid, per the shop tech’s preference at that moment. Beats counting them one by one.
Secure the lid and shake with all your might: you’re simulating radioactive decay! Loudly.
Pick a number from one to six. Say, three. Each die that turns up with three pips after a shake decays, and you remove it from the bin. With 180 dice in there, the chances of getting all threes – or zero threes – is vanishingly small. One-in-six raised to the 180th power, right? As a percentage that’s, what, nearly 140 zeros after the decimal point? Run the numbers, and you can look forward to around one-sixth of the dice in there decaying with each shake. Sometimes more, sometimes less.
You’ll also keep a close eye on those differently-colored dice. One for you, one for your partner. They’re the atoms you’re watching carefully, and unlike the sorta-predictable rolls of a large mass of dice, they’ll decay when they’re good and ready. Could be first, could be never. It’s an illustration of how probability works in systems of different sizes. Of how the random nature of radioactive decay produces a predictability with enough atoms and enough time.
In some idealized version of this experiment, you’d have 30 dice decay on the first shake. Then 25. Then 21. 17. 15. 12. 10. 8. 7. 6. 5. 4. 3. 3. 2. 2. 2. 1. 1. 1. After that… maybe one per shake? (The student experiment stops well before you’re down to a meager handful of dice.) The half-life arrives around four shakes. Every four shakes. Neat!
And should the effect with 180 dice not be enough? Compare your data to the rest of the lab, seeing how each rate of decay is nearly but not exactly the same. Then aggregate the data from all dozen lab benches. 2,160 dice decaying.
Loudly.

Greetings from one of the unofficial mascots of Physics, the drinking bird! Forever wearing its top hat, this classic toy is found all around the department. Though we keep a drawer full of them in storage, there are a handful about the shop shelves, professors’ offices, and occasionally elsewhere. The drinking bird is an example of a heat engine, which converts heat into mechanical energy.
Drinking birds are especially fun because they operate at room temperature. Two glass bulbs are connected by a tube and filled with methylene chloride, which has a low boiling point and condenses and vaporizes readily within the vessel. When the upright bird’s felt-covered head is wet, evaporative cooling causes a vapor pressure differential between the two ends. As liquid from the bottom rises, the bird becomes top-heavy and leans down for a drink, re-wetting the felt and priming the process to repeat. It’s an entertaining demonstration of the effects of various laws of physics.
There’s the ideal gas law, of course. Temperature change causes pressure change, which causes a shift in the balance of liquid and vapor. That shift in mass results in a center of mass that oscillates from one side to the other of the fulcrum, creating torque and movement. For as long as the bird can re-wet itself and maintain the temperature differential, the heat engine will continue to operate.
Alternately, you can apply a heat source to the lower bulb to get the same effect, which is the basis for most heat engines. There are many options to produce heat, and a wealth of engine designs to turn that thermal energy into useful work. But few are as simple, visually apparent, and entertaining as a bobbing glass toy.